

古脊椎动物学报 ›› 2023, Vol. 61 ›› Issue (2): 90-107.DOI: 10.19615/j.cnki.2096-9899.230217CSTR: 32090.14.j.cnki.2096-9899.230217
收稿日期:2023-01-04
出版日期:2023-04-20
发布日期:2023-04-18
通讯作者:
wangmin@ivpp.ac.cn基金资助:Received:2023-01-04
Published:2023-04-20
Online:2023-04-18
摘要:
反鸟类是目前已知物种多样性最丰富的中生代鸟类类群,已有超过60个反鸟类物种在近乎所有的大洲相继被发现,其存续时间贯穿整个白垩纪。多数反鸟类以及其他早期鸟翼类的化石材料多以二维平面的形式保存,而且鸟翼类头骨的骨骼大都轻薄而不易保存为化石,这些因素对研究早期鸟类头骨的形态学特征造成很大困难。早期鸟翼类头骨化石的稀缺极大地限制了关于恐龙相对笨重和非可动性的头骨与鸟类质轻而具有可动性的头骨之间如何演化的研究。报道了一件反鸟类渤海鸟科马氏副渤海鸟(Parabohaiornis martini)的新标本,并对其头骨形态进行了三维复原。研究结果显示副渤海鸟保留了原始的非鸟类恐龙所具有的颞区和腭区结构,进一步证实了上述头骨区域在演化上相对保守,以及多数鸟翼类原始类群仍然保留了非可动性头骨这一最近提出的假说。
中图分类号:
王敏. 马氏副渤海鸟(鸟翼类:反鸟类)新标本对鸟类头骨早期演化的意义. 古脊椎动物学报, 2023, 61(2): 90-107.
WANG Min. A new specimen of Parabohaiornis martini (Avialae: Enantiornithes) sheds light on early avian skull evolution. Vertebrata Palasiatica, 2023, 61(2): 90-107.

Fig. 1 Photograph of the new referred specimen of Parabohaiornis martini, IVPP V28398 Abbreviations: ald. alular digit; am. alular metacarpal; ca. caudal vertebra; ce. cervical vertebra;co. coracoid; dv. dorsal vertebra; fe. femur; fi. fibula; fu. furcula; hu. humerus; hy. hyoid; il. ilium; is. ischium;ma. major metacarpal; mad. major digit; mi. minor metacarpal; mid. minor digit; pu. pubis;py. pygostyle; p-I to p-IV. pedal digit I to IV; ra. radius; sc. scapula; sk. skull; st. sternum; sy. synsacrum;ti. tibiotarsus; tm. tarsometatarsus; un. ulna; /r(l). right (left) side. Scale bar = 20 mm

Fig. 2 Detailed postcranial features of the referred specimen of Parabohaiornis martini, IVPP V28398 A. pectoral region; B. distal hindlimb Abbreviations: ci. capital incisure; m-I to m-IV. metatarsal I to IV; other abbreviations seen in Fig. 1 Anatomical features used to diagnose this specimen as Parabohaiornis martini are numbered: 1. omal ends of the scapula expanded; 2. acromion process of the scapula extending proximally in parallel to the shaft lateral view; 3. pygostyle without an abrupt distal constriction;4. pedal digit II much robust than other pedal digits. Scale bars = 20 mm
| Specimen (length) | V18691 | V18690 | V28398 |
|---|---|---|---|
| Skull | 42.5 | 44.1 | |
| Coracoid | 21.9 | 25.6 | 28.3* |
| Scapula | 33.3 | 41.2 | |
| Humerus | 43.4 | 46.7 | 52.5 |
| Ulna | 43.8 | 55.7 | |
| Radius | 40.3 | 52.7* | |
| Alular metacarpal | 3,9 | 4.2 | |
| Alular digit 1 | 8.1 | 9.5 | |
| Alular digit 2 | 3.5 | 4.3 | |
| Major digit 1 | 10.2 | 10.9 | |
| Major digit 2 | 7.4 | 7.8 | |
| Major digit 3 | 3.2 | 3.5 | |
| Minor digit 1 | 5.5 | 5.8 | |
| Pygostyle | 18.0 | 21.8 | 24.1* |
| Tibiotarsus | 40.0 | 45.0 | 54.4 |
| Metatarsal II | 17.1 | 20.4 | 23.7 |
| Metatarsal III | 19.5 | 22.0 | 26.7 |
| Metatarsal IV | 18.1 | 20.6 | 24.3 |
| Digit I-1 | 5.4 | 6.1 | 6.1 |
| Digit I-2 | 6.0 | 6.3* | 6.8 |
| Digit II-1 | 4.5 | 5.8 | 6.2 |
| Digit II-2 | 6.8 | 7.6 | 8.5 |
| Digit III-1 | 6.4 | 7.8 | 7.6 |
| Digit III-2 | 5.4 | 6.5 | 8.2 |
| Digit IV-1 | 2.9 | 2.9 | 3.7 |
| Digit IV-2 | 2.6 | 2.6 | 3.4 |
| Digit IV-3 | 2.9 | 2.9 | 3.4 |
| Digit IV-4 | 4.1 | 4.4 | 4.3 |
Table 1 Selected measurements of IVPP V28398 in comparison with other Parabohaiornis martini (V18691: holotype; V18690: referred specimen; data from Wang et al., 2014a) (mm)
| Specimen (length) | V18691 | V18690 | V28398 |
|---|---|---|---|
| Skull | 42.5 | 44.1 | |
| Coracoid | 21.9 | 25.6 | 28.3* |
| Scapula | 33.3 | 41.2 | |
| Humerus | 43.4 | 46.7 | 52.5 |
| Ulna | 43.8 | 55.7 | |
| Radius | 40.3 | 52.7* | |
| Alular metacarpal | 3,9 | 4.2 | |
| Alular digit 1 | 8.1 | 9.5 | |
| Alular digit 2 | 3.5 | 4.3 | |
| Major digit 1 | 10.2 | 10.9 | |
| Major digit 2 | 7.4 | 7.8 | |
| Major digit 3 | 3.2 | 3.5 | |
| Minor digit 1 | 5.5 | 5.8 | |
| Pygostyle | 18.0 | 21.8 | 24.1* |
| Tibiotarsus | 40.0 | 45.0 | 54.4 |
| Metatarsal II | 17.1 | 20.4 | 23.7 |
| Metatarsal III | 19.5 | 22.0 | 26.7 |
| Metatarsal IV | 18.1 | 20.6 | 24.3 |
| Digit I-1 | 5.4 | 6.1 | 6.1 |
| Digit I-2 | 6.0 | 6.3* | 6.8 |
| Digit II-1 | 4.5 | 5.8 | 6.2 |
| Digit II-2 | 6.8 | 7.6 | 8.5 |
| Digit III-1 | 6.4 | 7.8 | 7.6 |
| Digit III-2 | 5.4 | 6.5 | 8.2 |
| Digit IV-1 | 2.9 | 2.9 | 3.7 |
| Digit IV-2 | 2.6 | 2.6 | 3.4 |
| Digit IV-3 | 2.9 | 2.9 | 3.4 |
| Digit IV-4 | 4.1 | 4.4 | 4.3 |
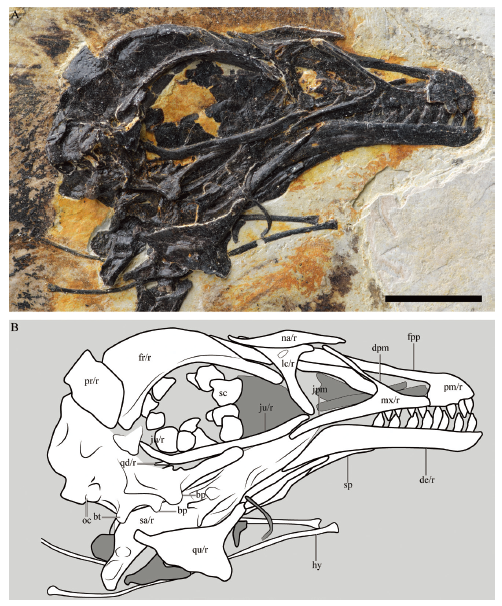
Fig. 3 Photograph (A) and line drawing (B) of the cranial anatomy of the referred specimen of Parabohaiornis martini, IVPP V28398 Abbreviations: bp. basipterygoid process; bt. basal tubera; de. dentary; dpm. dorsal process of maxilla; fpp. frontal process of premaxilla; fr. frontal; hy. hyoid; jpm. jugal process of maxilla; ju. jugal; lc. lacrimal; mx. maxilla; na. nasal; oc. occipital condyle; pm. premaxilla; pr. parietal; qd. quadratojugal; qu. quadrate; sa. surangular-articular; sc. scleral ossicle; sp. splenial; /r(l). right (left) side. Scale bar = 10 mm

Fig. 4 Cranial anatomy of the referred specimen of Parabohaiornis martini, IVPP V28398 CT scanning of the skull in right lateral (A) and left lateral (B) views The orange arrowheads denote the four premaxillary teeth on the left side; the red arrowhead indicates the fused proximal ends of the frontal processes of premaxilla that are separated distally (blue arrowhead) Abbreviations: ag. angular; po. postorbital; sq. squamosal; other abbreviations seen in Fig. 3 Scale bars = 10 mm
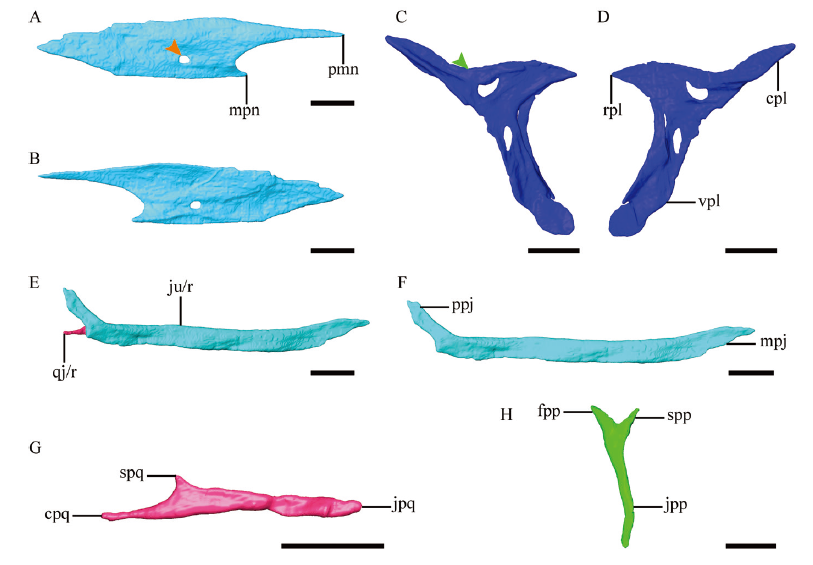
Fig. 5 Anatomy of selected cranial bones of Parabohaiornis martini, IVPP V28398 Digital reconstruction of the right nasal in lateral (A) and medial (B) views, right lacrimal in lateral (C) and medial (D) views, overlapping right jugal and quadratojugal in lateral view (E), right jugal in lateral view (F), right quadratojugal in medial view and mirrored (G), and left postorbital in lateral view (H) The orange arrowhead in A denotes the foramen in the nasal, and the green arrowhead in C indicates the concave dorsal margin of the lacrimal Abbreviations: cpl. caudal process of lacrimal; cpq. caudal process of quadratojugal; fpp. frontal process of postorbital; ju. jugal; jpp. jugal process of postorbital; jpq. jugal process of quadratojugal; mpj. maxillary process of jugal; mpn. maxillary process of nasal; pmn. premaxillary process of nasal; ppj. postorbital process of jugal; rpl. rostral process of lacrimal; spp. squamosal process of postorbital; spq. squamosal process of quadratojugal; vpl. ventral process of lacrimal; /r(l). right (left) side. Scale bars = 2 mm
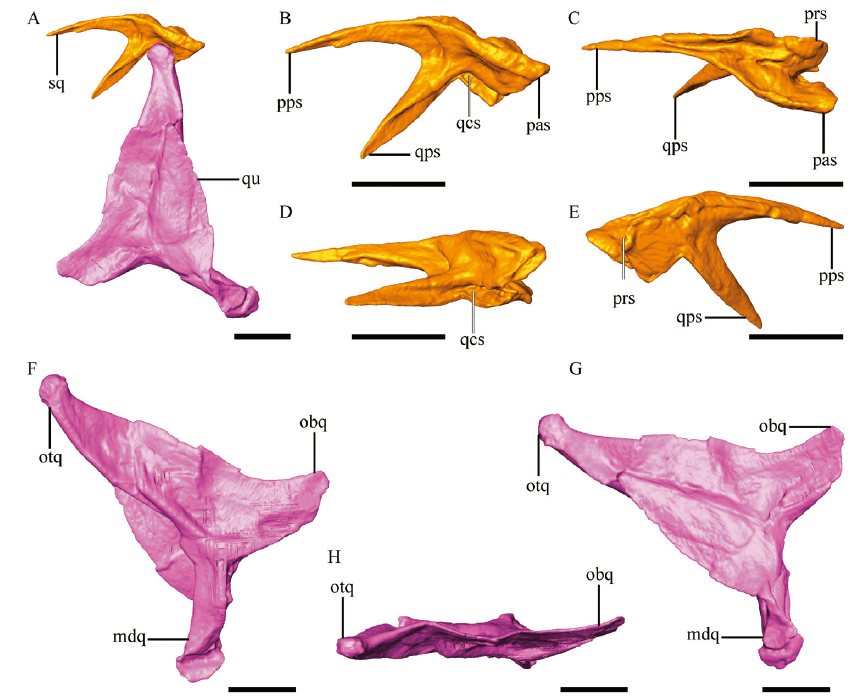
Fig. 6 Anatomy of temporal bones of Parabohaiornis martini, IVPP V28398 Digital reconstruction of the articulated left squamosal and quadrate (A), left squamosal in lateral (B), dorsal (C), ventral (D), medial (E) views, and left quadrate in rostrolateral (F), caudomedial (G), dorsal (H) views Abbreviations: mdq. mandible process of quadrate; obq. orbital process of quadrate; otq. otic process of quadrate; pas. paraoccipital process of squamosal; pps. postorbital process of squamosal; prs. parietal process of squamosal; qcs. quadrate cotyla of squamosal; qps. quadratojugal process of squamosal; qu. quadrate; sq. squamosal. Scale bars = 2 mm
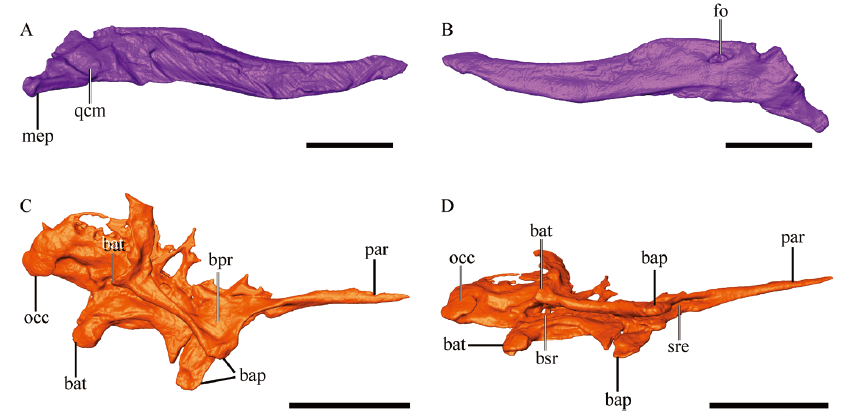
Fig. 7 Anatomy of selected elements of lower jaw and cranial base of Parabohaiornis martini, IVPP V28398 Digital reconstruction of the left surangular-articular in medial (A) and lateral (B) views,basioccipital-basisphenoid-parasphenoid in lateral (C) and ventral (D) views Abbreviations: bap. basipterygoid process; bat. basal tubera; bpr. basipterygoid recess; bsr. basisphenoid recess; fo. lateral fossa of surangular; mep. medial process of mandible; occ. occipital condyle;par. parasphenoid rostrum; qcm. quadrate cotyle of mandible; sre. subsellar recess. Scale bars = 5 mm
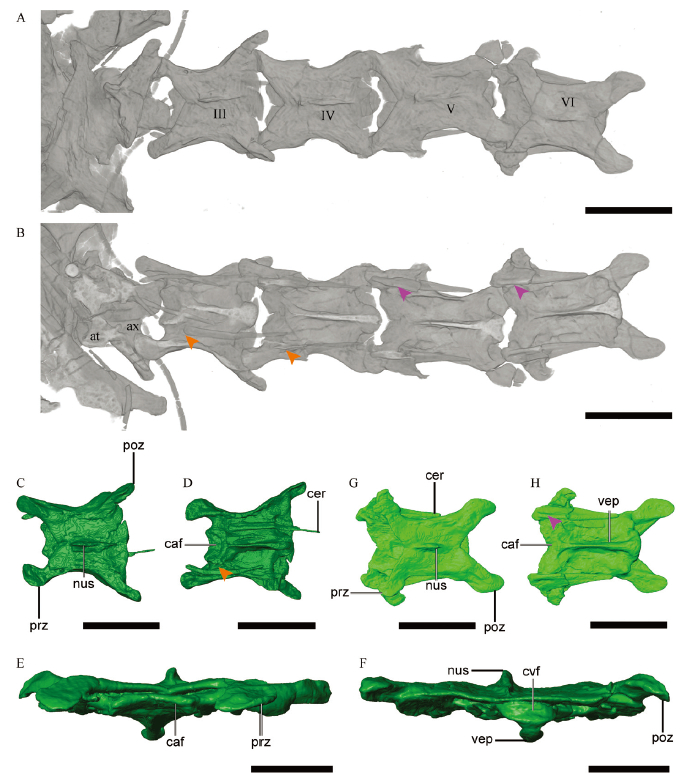
Fig. 8 Anatomy of cranial cervical vertebrae of Parabohaiornis martini, IVPP V28398 CT image of the cranialmost six cervicals in dorsal (A) and ventral (B) views, respectively. Digital reconstruction of the third cervical in dorsal (C), ventral (D), cranial (E) and caudal (F) views; the sixth cervical in dorsal (G) and ventral (H) views. The orange arrowheads in B and D denote the unfused cervical ribs and centra, and the purple arrowheads in B and H denote the fusion between the cervical ribs and centra Abbreviations: at. atlas; ax. axis; caf. cranial articular facet of centrum; cer. cervical rib; cvf. caudal articular facet of centrum; nus. neural spine; poz. postzygapophysis; prz. prezygapophysis; vep. ventral process;III-VI. third to sixth cervicals. Scale bars = 5 mm (A-D, G, H); scale bars = 2 mm (E, F)
| [1] | Barsbold R, 1974. Saurornithoididae, a new family of small theropod dinosaurs from central Asia and North America. Palaeontol Pol, 30: 5-22 |
| [2] | Barsbold R, Osmólska H, 1999. The skull of Velociraptor (Theropoda) from the Late Cretaceous of Mongolia. Acta Palaeontol Pol, 44: 189-219 |
| [3] | Baumel J J, Witmer L M, Cambridge, 1993. Osteologia. In: Baumel J J, King A S, Breazile J E et al. eds. Handbook of Avian Anatomy: Nomina Anatomica Avium, 2nd ed. UK: Nuttall Ornithological Club. 45-132 |
| [4] | Bertelli S, Giannini N P, Ksepka D T, 2006. Redescription and phylogenetic position of the Early Miocene penguin Paraptenodytes antarcticus from Patagonia. Am Mus Novit, 36: 1-36 |
| [5] |
Bock W J, 1964. Kinetics of the avian skull. J Morphol, 114: 1-41
DOI URL |
| [6] |
Chiappe L M, Ji S A, Ji Q, 2007. Juvenile birds from the Early Cretaceous of China: implications for enantiornithine ontogeny. Am Mus Novit, 3594: 1-46
DOI URL |
| [7] |
Chiappe L M, Meng Q J, Serrano F et al., 2019. New Bohaiornis-like bird from the Early Cretaceous of China: enantiornithine interrelationships and flight performance. PeerJ, 7: e7846
DOI URL |
| [8] |
Currie P J, 1995. New information on the anatomy and relationships of Dromaeosaurus albertensis (Dinosauria: Theropoda). J Vert Paleont, 15: 576-591
DOI URL |
| [9] |
Currie P J, Zhao X J, 1993. A new carnosaur (Dinosauria, Theropoda) from the Jurassic of Xinjiang, People’s Republic of China. Can J Earth Sci, 30: 2037-2081
DOI URL |
| [10] | Elżanowski A, 1991. New observations of the skull of Hesperornis with reconstructions of the bony palate and otic region. Postilla, 207: 1-20 |
| [11] |
Elżanowski A, Stidham T A, 2011. A galloanserine quadrate from the Late Cretaceous Lance Formation of Wyoming. Auk, 128: 138-145
DOI URL |
| [12] |
Elżanowski A, Wellnhofer P, 1996. Cranial morphology of Archaeopteryx: evidence from the seventh skeleton. J Vert Paleont, 16: 81-94
DOI URL |
| [13] | Gingerich P D, 1976. Evolutionary significance of the Mesozoic toothed birds. Smithson Contrib Paleobiol, 27: 23-33 |
| [14] |
Gussekloo S W S, Berthaume M A, Pulaski D R et al., 2017. Functional and evolutionary consequences of cranial fenestration in birds. Evolution, 71: 1327-1338
DOI PMID |
| [15] |
Hendrickx C, Araújo R, Mateus O, 2015. The non-avian theropod quadrate I: standardized terminology with an overview of the anatomy and function. PeerJ, 3: e1245
DOI URL |
| [16] |
Holliday C M, Witmer L M, 2008. Cranial kinesis in dinosaurs: intracranial joints, protractor muscles, and their significance for cranial evolution and function in diapsids. J Vert Paleont, 28: 1073-1088
DOI URL |
| [17] |
Hu D Y, Li L, Hou L H et al., 2011. A new enantiornithine bird from the Lower Cretaceous of western Liaoning, China. J Vert Paleont, 31: 154-161
DOI URL |
| [18] |
Hu H, O’Connor J K, Wang M et al., 2020. New anatomical information on the bohaiornithid Longusunguis and the presence of a plesiomorphic diapsid skull in Enantiornithes. J Syst Palaeontol, 18: 1481-1495
DOI URL |
| [19] |
Li Z H, Wang M, Stidham T A et al., 2023. Decoupling the skull and skeleton in a Cretaceous bird with unique appendicular morphologies. Nat Ecol Evol, 7: 20-31
DOI PMID |
| [20] |
Livezey B C, Zusi R L, 2006. Higher-order phylogeny of modern birds (Theropoda, Aves: Neornithes) based on comparative anatomy: I. methods and characters. Bull Carnegie Mus Nat Hist, 37: 1-544
DOI URL |
| [21] | Livezey B C, Zusi R L, 2007. Higher-order phylogeny of modern birds (Theropoda, Aves: Neornithes) based on comparative anatomy: II. analysis and discussion. Zool J Linn Soc, 149: 1-95 |
| [22] | Lovette I J, Fitzpatrick J W, 2016. Handbook of Bird Biology, 3rd ed. Chichester: John Wiley & Sons. 1-716 |
| [23] | Lucas F A, 1903. Notes on the osteology and relationship of the fossil birds of the genera Hesperornis, Hargeria, Baptornis, and Diatryma. Proc US Natn Mus, 26: 545-556 |
| [24] | McDowell S, 1948. The bony palate of birds. Part I. The Palaeognathae. Auk, S: 520-549 |
| [25] |
Norell M A, Clark J M, Turner A H et al., 2006. A new dromaeosaurid theropod from Ukhaa Tolgod (Ömnögov, Mongolia). Am Mus Novit, 3545: 1-51
DOI URL |
| [26] |
O’Connor J K, Chiappe L M, 2011. A revision of enantiornithine (Aves: Ornithothoraces) skull morphology. J Syst Palaeontol, 9: 135-157
DOI URL |
| [27] | O’Connor J K, Zhang Y G, Chiappe L M et al., 2013. A new enantiornithine from the Yixian Formation with the first recognized avian enamel specialization. J Vert Paleont, 33: 1-12 |
| [28] |
O’Connor P M, Turner A H, Groenke J R et al., 2020. Late Cretaceous bird from Madagascar reveals unique development of beaks. Nature, 588: 272-276
DOI |
| [29] | Ostrom J H, 1969. Osteology of Deinonychus antirrhopus, an unusual theropod from the Lower Cretaceous of Montana. Bull Peabody Mus Nat Hist, 30: 1-165 |
| [30] | Rauhut O W M, 2003. The interrelationships and evolution of basal theropods. Spec Pap Palaeontol, 69: 1-213 |
| [31] |
Rauhut O W M, 2014. New observations on the skull of Archaeopteryx. Paläontol Z, 88: 211-221
DOI URL |
| [32] |
Stidham T A, O’Connor J K, 2021. The evolutionary and functional implications of the unusual quadrate of Longipteryx chaoyangensis (Avialae: Enantiornithes) from the Cretaceous Jehol Biota of China. J Anat, 239: 1066-1074
DOI PMID |
| [33] |
Sullivan C, Xu X, 2017. Morphological diversity and evolution of the jugal in dinosaurs. Anat Rec, 300: 30-48
DOI URL |
| [34] |
Wang M, Hu H, 2017. A comparative morphological study of the jugal and quadratojugal in early birds and their dinosaurian relatives. Anat Rec, 300: 62-75
DOI URL |
| [35] | Wang M, Zhou Z H, O’Connor J K et al., 2014a. A new diverse enantiornithine family (Bohaiornithidae fam. nov.) from the Lower Cretaceous of China with information from two new species. Vert PalAsiat, 52: 31-76 |
| [36] |
Wang M, O’Connor J K, Zhou Z H, 2014b. A new robust enantiornithine bird from the Lower Cretaceous of China with scansorial adaptations. J Vert Paleont, 34: 657-671
DOI URL |
| [37] |
Wang M, Stidham T A, Li Z H et al., 2021. Cretaceous bird with dinosaur skull sheds light on avian cranial evolution. Nat Commun, 12: 3890
DOI PMID |
| [38] |
Wang M, Stidham T A, O’Connor J K et al., 2022. Insight into the evolutionary assemblage of cranial kinesis from a Cretaceous bird. eLife, 11: e81337
DOI URL |
| [39] |
Wang X R, O’Connor J K, Zhao B et al., 2010. New species of Enantiornithes (Aves: Ornithothoraces) from the Qiaotou Formation in Northern Hebei, China. Acta Geol Sin, 84: 247-256
DOI URL |
| [40] | Weishampel B D, Dodson P, Osmólska H, 2004. The Dinosauria, 2nd ed. Berkeley: University of California Press. 1-880 |
| [41] | Witmer L, 1997. Craniofacial air sinus systems. In: Currie P J, Padian K eds. Encyclopedia Dinosauria. New York: Academic Press. 1-159 |
| [42] |
Yu Z Q, Wang M, Li Y et al., 2021. New geochronological constraints for the Lower Cretaceous Jiufotang Formation in Jianchang basin, NE China, and their implications for the late Jehol Biota. Palaeogeogr Palaeoclimatol Palaeoecol, 583: 110657
DOI URL |
| [43] |
Zhou Z H, Luis M C, Zhang F C, 2005. Anatomy of the Early Cretaceous bird Eoenantiornis buhleri (Aves : Enantiornithes) from China. Can J Earth Sci, 42: 1331-1338
DOI URL |
| [44] |
Zhou Z H, Clarke J, Zhang F C, 2008. Insight into diversity, body size and morphological evolution from the largest Early Cretaceous enantiornithine bird. J Anat, 212: 565-577
DOI PMID |
| [45] |
Zhang Z H, Chiappe L M, Han G et al., 2013. A large bird from the Early Cretaceous of China: new information on the skull of enantiornithines. J Vert Paleont, 33: 1176-1189
DOI URL |
| [46] | Zusi R L, 1984. A functional and evolutionary analysis of rhynchokinesis in birds. Smithson Contrib Zool, 395: 1-40 |
| [1] | 徐冉成, 李茜. 内蒙古乌兰塔塔尔地区早渐新世梳趾鼠类头骨新材料. 古脊椎动物学报, 2020, 58(4): 305-327. |
| [2] | 邓 涛,张云翔,曾志杰,侯素宽. 记恐剑齿虎一头骨及剑齿虎镶嵌进化中体型巨大化的新证据. 古脊椎动物学报, 2016, 54(4): 302-318. |
| [3] | 胡 晗,周忠和,邹晶梅 . 鹏鸟(Pengornis)一新材料及其对反鸟特征演化的指示意义. 古脊椎动物学报, 2014, 52(1): 77-97. |
| [4] | 王 敏,周忠和,邹晶梅,Nikita V. ZELENKOV. 中国早白垩世反鸟类一新科 (Bohaiornithidae fam. nov.). 古脊椎动物学报, 2014, 52(1): 31-76. |
| [5] | 邹晶梅. 对长趾辽宁鸟的重新认识. 古脊椎动物学报, 2012, 50(1): 25-37. |
| [6] | 李萍,王元青. 内蒙古中部新发现的Schlosseria magister头骨材料. 古脊椎动物学报, 2010, 48(2): 119-132. |
| [7] | 伍少远. Alloptox gobiensis (兔形目,鼠兔科) 头骨形态及系统位置. 古脊椎动物学报, 2003, 41(02): 115-130. |
| [8] | 张法奎, 杜湘珂, 朱奇志, 程希侃. 完美中国颌兽(Sinognathus gracilis)头骨化石标本的新观察. 古脊椎动物学报, 1999, 37(04): 267-277. |
| [9] | 侯连海, 拉里•马丁, 周忠和, 艾伦•费多契亚. 中国发现从始祖鸟到反鸟的重要缺失环节. 古脊椎动物学报, 1999, 37(02): 88-95. |
| [10] | 叶捷, 邱占祥, 陈景智. 记同心铲齿象一幼年头骨化石. 古脊椎动物学报, 1989, 27(04): 1-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
