

Vertebrata Palasiatica ›› 2024, Vol. 62 ›› Issue (2): 99-119.DOI: 10.19615/j.cnki.2096-9899.240305
Previous Articles Next Articles
LIU Bi-Ying1,2, Thomas A. STIDHAM1,2, WANG Xiao-Ping3, LI Zhi-Heng1,*( ), ZHOU Zhong-He1,2
), ZHOU Zhong-He1,2
Received:2023-11-07
Online:2024-04-20
Published:2024-05-08
Contact:
*lizhiheng@ivpp.ac.cn
刘碧莹1,2, Thomas A. STIDHAM1,2, 王小平3, 李志恒1,*( ), 周忠和1,2
), 周忠和1,2
基金资助:CLC Number:
LIU Bi-Ying, Thomas A. STIDHAM, WANG Xiao-Ping, LI Zhi-Heng, ZHOU Zhong-He. Morphometric analysis of the cervical vertebral series in extant birds with implications for Mesozoic avialan feeding ecology. Vertebrata Palasiatica, 2024, 62(2): 99-119.
刘碧莹, Thomas A. STIDHAM, 王小平, 李志恒, 周忠和. 2024, 62(2): 99-119, 现生鸟类颈椎形态测定分析及其对中生代鸟类饮食生态的启示. 古脊椎动物学报.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.vertpala.ac.cn/EN/10.19615/j.cnki.2096-9899.240305
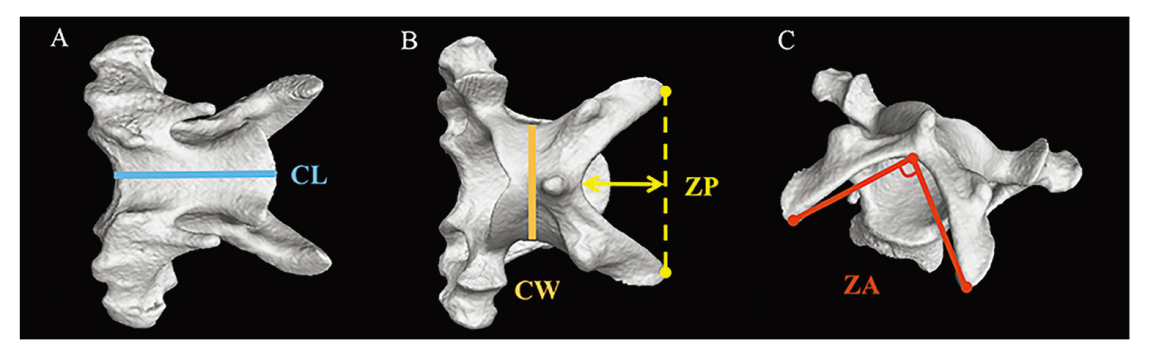
Fig. 1 Measurements acquired for individual cervical vertebrae The fifth cervical vertebra of Falco subbuteo was used as an exemplar here as showing in ventral (A), dorsal (B), and dorsal-caudal (C) views with measurement axes and angles Abbreviations: CL. centrum length; CW. centrum width; ZA. zygapophyseal angle;ZP. zygapophoseal protrusion
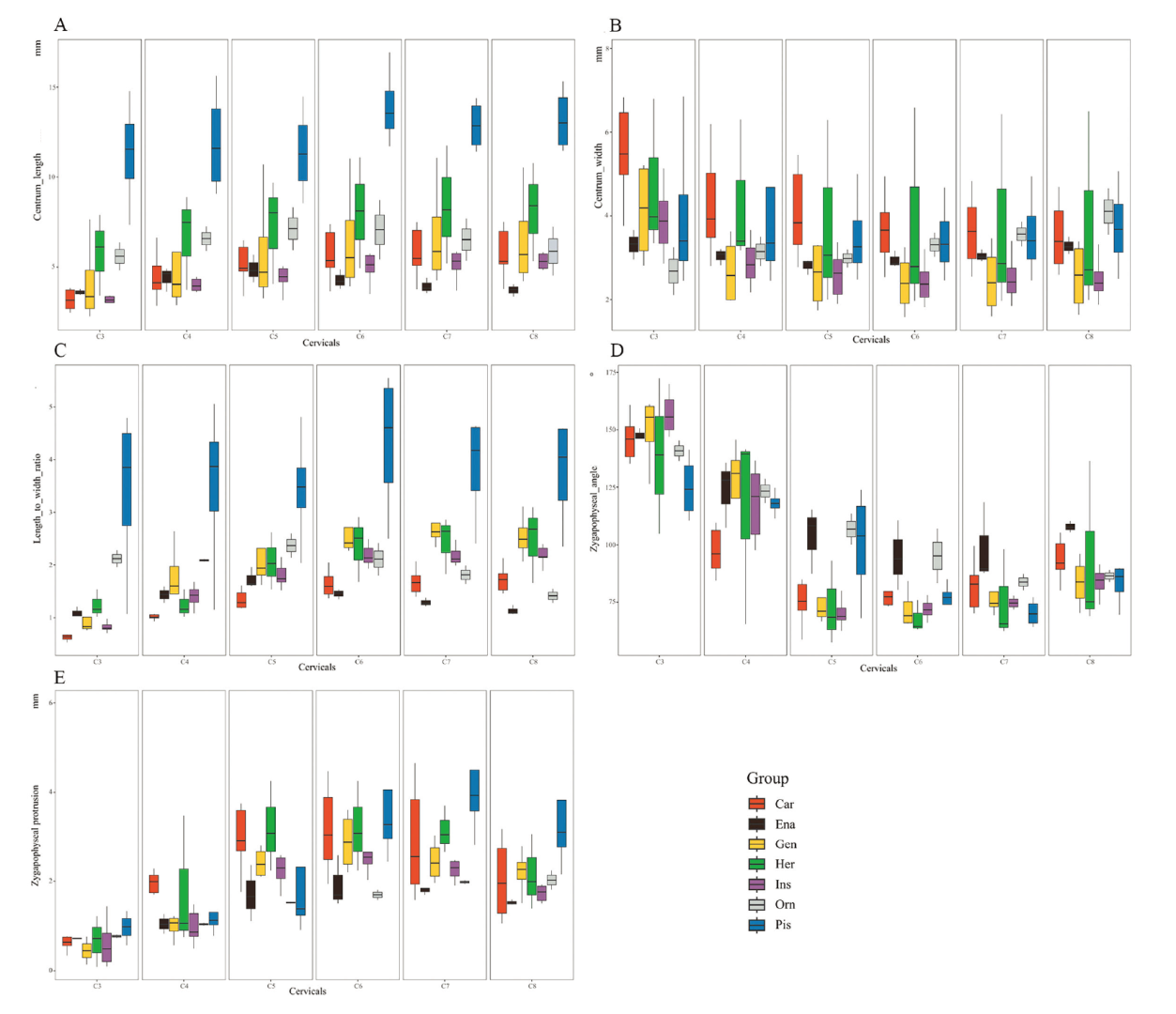
Fig. 2 Comparison of raw measurements from sampled birds The data covers cervical vertebra from C3 to C8. Box plots showing the comparison of cervical change in length (A), width (B), ratio of centrum length to width (C), zygapophyseal angle (D), and zygapophoseal protrusion (E). The black line in the box represents the median value of the measurements for given group, and the endpoint of the upper and lower whiskers represent the maximum and minimum values, respectively Abbreviations: Car. carnivory (red); Ena. enantiornithine (black); Gen. generalist (yellow);Her. herbivore (green); Ins. insectivore (purple); Orn. ornithurine (gray); Pis. piscivore (blue)
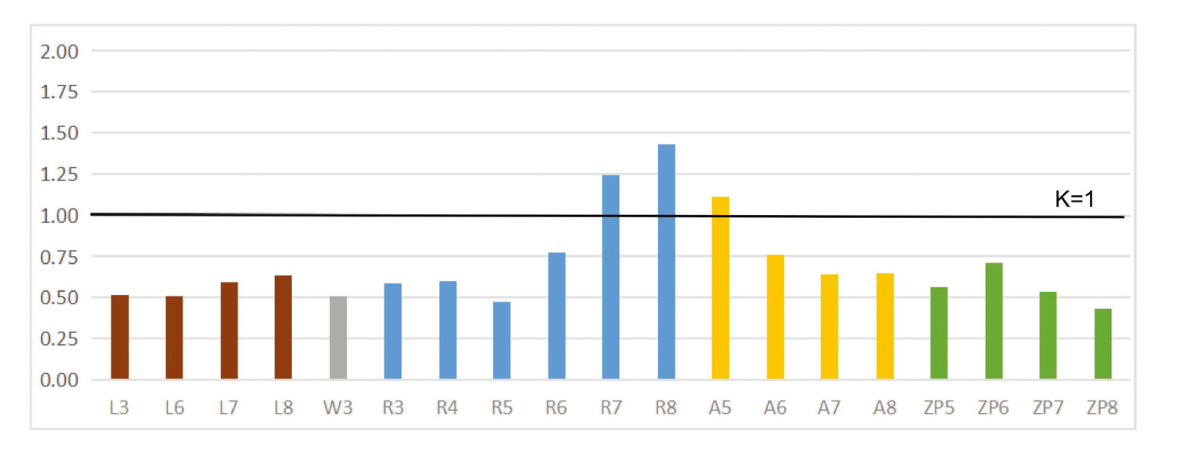
Fig. 3 Significant phylogenetic signal detected in cervical vertebral traits using Blomberg’s K Traits with K-value over or equal one indicates strong phylogenetic signal as following browning motion and are phylogenetically conserved. Traits with a K-value smaller than one indicate a departure from Brownian motion. Different categories of measurements are labeled with different colors Abbreviations: A. zygapophyseal angle; L. centrum length; R. ratio of centrum length to width;W. centrum width; ZP. zygapophoseal protrusion
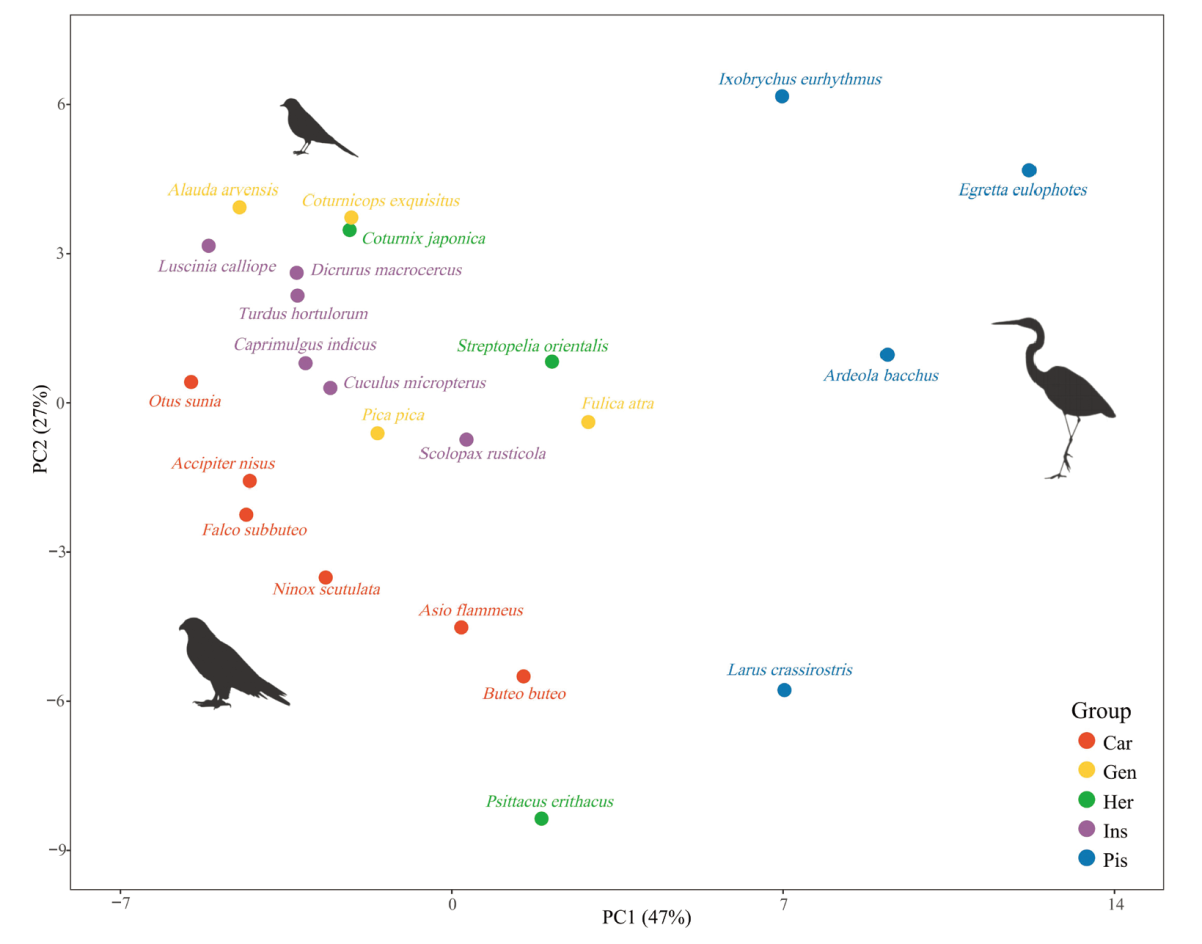
Fig. 4 PCA of the cervical measurements for extant birds only, C3 to C12 covered Abbreviations see Fig. 2. See online supplementary file 1 for details on loadings and percentages of variance explained (Fig. S2)
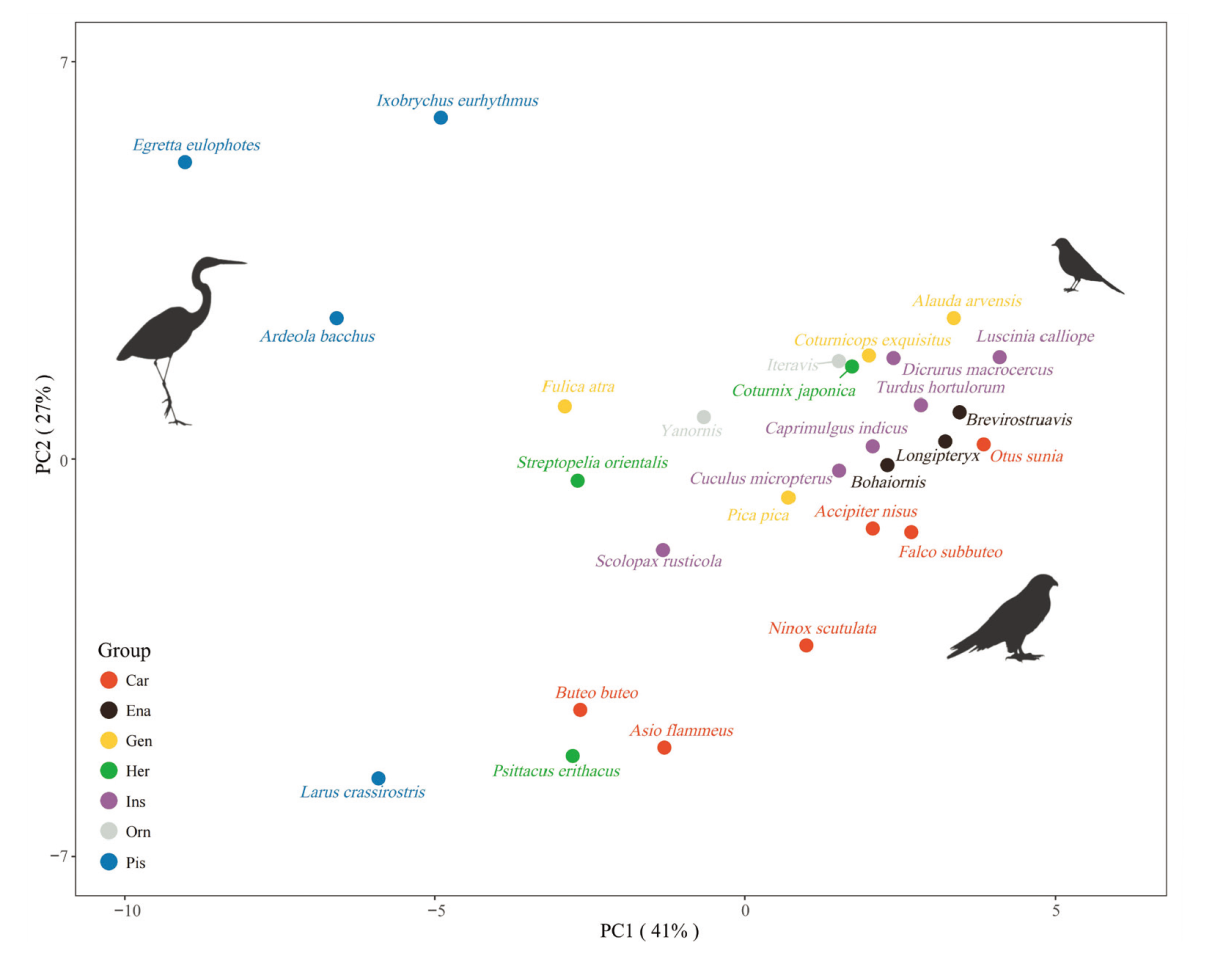
Fig. 5 PCA results with both extant and extinct birds, C3 to C8 covered Abbreviations see Fig. 2. See online supplementary file 1 for details on loadings and percent variance explained (Fig. S3)
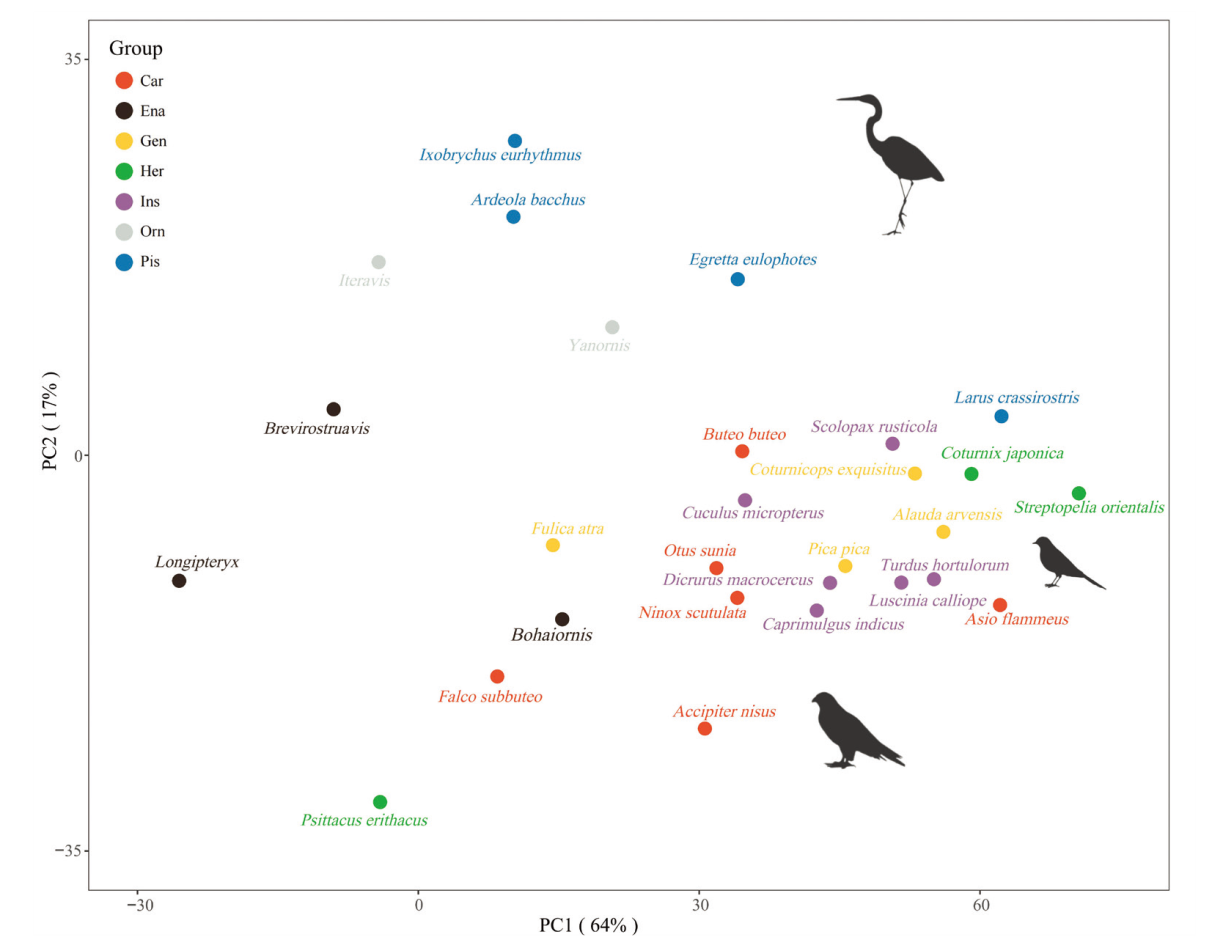
Fig. 6 Phylogenetic PCA for extant and extinct birds with data for vertebrae C3 to C8 Variables in Fig. 3 were involved with this pPCA analysis. Abbreviations see Fig. 2
| [1] |
Blomberg S P, Garland T Jr, Ives A R, 2003. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution, 57: 717-745
DOI PMID |
| [2] | Böhmer C, Rauhut O W M, Wörheide G, 2015. Correlation between Hox code and vertebral morphology in archosaurs. Proc R Soc B-Biol Sci, 282: 20150077 |
| [3] |
Böhmer C, Plateau O, Cornette R et al., 2019. Correlated evolution of neck length and leg length in birds. R Soc Open Sci, 6: 181588
DOI URL |
| [4] | Chiappe L, Walker C, 2002. Skeletal morphology and systematics of the Cretaceous Euenantiornithes (Ornithothoraces:Enantiornithes). In: ChiappeL M, WitmerL M, eds. Mesozoic Birds:Above the Heads of Dinosaurs. Berkeley: University of California Press. 240-267 |
| [5] |
Cobley M J, Rayfield E J, Barrett P M, 2013. Inter-vertebral flexibility of the ostrich neck: implications for estimating sauropod neck flexibility. PLoS One, 8: e72187
DOI URL |
| [6] |
Cooney C R, Bright J A, Capp E J R et al., 2017. Mega-evolutionary dynamics of the adaptive radiation of birds. Nature, 542: 344-347
DOI |
| [7] |
Falk A R, Lamsdell J C, Gong E, 2021. Principal component analysis of avian hind limb and foot morphometrics and the relationship between ecology and phylogeny. Paleobiology, 47: 314-336
DOI URL |
| [8] |
Freckleton R P, Harvey P H, Pagel M, 2002. Phylogenetic analysis and comparative data: a test and review of evidence. Am Nat, 160: 712-726
DOI URL |
| [9] |
Harmon L J, Weir J T, Brock C D et al., 2007. GEIGER: investigating evolutionary radiations. Bioinformatics, 24: 129-131
DOI URL |
| [10] |
Heidweiller J, 1989. Post natal development of the neck system in the chicken (Gallus domesticus). Am J Anat, 186: 258-270
DOI URL |
| [11] | Hu H, Zhou Z H, O’Connor J K et al., 2014. A subadult specimen of Pengornis and character evolution in Enantiornithes. Vert PalAsiat, 52(1): 77-97 |
| [12] |
Kambic R E, Biewener A A, Pierce S E, 2017. Experimental determination of three-dimensional cervical joint mobility in the avian neck. Front Zool, 14: 37
DOI PMID |
| [13] | Kamilar J M, Cooper N, 2013. Phylogenetic signal in primate behaviour, ecology and life history. Philos Trans R Soc B-Biol Sci, 368: 20120341 |
| [14] | Kardong K V, 2012. Vertebrates:Comparative Anatomy, Function, Evolution. New York: McGraw-Hill. 294-324 |
| [15] |
Kembel S W, Cowan P D, Helmus M R et al., 2010. Picante: R tools for integrating phylogenies and ecology. Bioinformatics, 26: 1463-1464
DOI PMID |
| [16] |
Krings M, Nyakatura J A, Fischer M S et al., 2014. The cervical spine of the American Barn Owl (Tyto furcata pratincola): I. Anatomy of the vertebrae and regionalization in their S-shaped arrangement. PLoS One, 9: e91653
DOI URL |
| [17] |
Krings M, Nyakatura J A, Boumans M L L M et al., 2017. Barn owls maximize head rotations by a combination of yawing and rolling in functionally diverse regions of the neck. J Anat, 231: 12-22
DOI PMID |
| [18] |
Li Z H, Clarke J A, 2016. The craniolingual morphology of waterfowl (Aves, Anseriformes) and its relationship with feeding Mode revealed through contrast-enhanced X-Ray computed tomography and 2D morphometrics. Evol Biol, 43: 12-25
DOI URL |
| [19] |
Li Z H, Zhou Z H, Wang M et al., 2014. A new specimen of large-bodied basal Enantiornithine Bohaiornis from the Early Cretaceous of China and the inference of feeding ecology in Mesozoic birds. J Paleontol, 88: 99-108
DOI URL |
| [20] |
Li Z H, Wang M, Stidham T A et al., 2022. Novel evolution of a hyper-elongated tongue in a Cretaceous enantiornithine from China and the evolution of the hyolingual apparatus and feeding in birds. J Anat, 240: 627-638
DOI URL |
| [21] | Liu Y, Chen S, 2021. The CNG Field Guide to the Birds of China. Changsha: Hunan Science and Technology Press. 1-636 |
| [22] |
Marek R D, 2023. A surrogate forelimb: evolution, function and development of the avian cervical spine. J Morphol, 284: e21638
DOI URL |
| [23] | Marek R D, Falkingham P L, Benson R B J et al., 2021. Evolutionary versatility of the avian neck. Proc R Soc B-Biol Sci, 288: 20203150 |
| [24] |
Miller C V, Pittman M, 2021. The diet of early birds based on modern and fossil evidence and a new framework for its reconstruction. Biol Rev, 96: 2058-2112
DOI URL |
| [25] |
Miller C V, Pittman M, Wang X et al., 2023. Quantitative investigation of pengornithid enantiornithine diet reveals macrocarnivorous ecology evolved in birds by Early Cretaceous. iScience, 26: 106211
DOI URL |
| [26] |
O’Connor J K, 2019. The trophic habits of early birds. Palaeogeogr, Palaeoclimatol, Palaeoecol, 513: 178-195
DOI URL |
| [27] |
O’Connor J K, Chiappe L, 2011. A revision of enantiornithine (Aves: Ornithothoraces) skull morphology. J Syst Palaeontol, 9: 135-157
DOI URL |
| [28] |
O’Connor J K, Wang X R, Chiappe L et al., 2009. Phylogenetic support for a specialized clade of Cretaceous Enantiornithine birds with information from a new species. J Vert Paleont, 29: 188-204
DOI URL |
| [29] |
Paradis E, Claude J, Strimmer K, 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics, 20: 289-290
DOI PMID |
| [30] |
Revell L J, 2009. Size-correction and principal components for interspecific comparative studies. Evolution, 63: 3258-3268
DOI PMID |
| [31] |
Revell L J, 2012. Phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol Evol, 3: 217-223
DOI URL |
| [32] | Rico-Guevara A, Sustaita D, Gussekloo S et al., 2019. Feeding in birds:thriving in terrestrial, aquatic, and aerial niches. In: BelsV, Whishaw I eds. Cham: Springer. 643-693 |
| [33] |
Tambussi C P, de Mendoza R, Degrange F J et al., 2012. Flexibility along the neck of the Neogene terror bird Andalgalornis steulleti (Aves Phorusrhacidae). PLoS One, 7: e37701
DOI URL |
| [34] |
Terray L, Plateau O, Abourachid A et al., 2020. Modularity of the neck in birds (Aves). Evol Biol, 47: 97-110
DOI |
| [35] | Upchurch P, Barrett P M, 2000. The evolution of sauropod feeding mechanisms. In: Sues H D ed. Evolution of Herbivory in Terrestrial Vertebrates:Perspectives from the Fossil Record. Cambridge: Cambridge University Press. 79-122 |
| [36] | van der Leeuw A H J, Bout R G, Zweers G A, 2015. Control of the cranio-cervical system during feeding in birds. Am Zool, 41: 1352-1363 |
| [37] | Venables B, Ripley B, 2002. Modern Applied Statistics With S. New York: Springer. 1-465 |
| [38] | Wang M, 2023. A new specimen of Parabohaiornis martini (Avialae: Enantiornithes) sheds light on early avian skull evolution. Vert PalAsiat, 61(2): 90-107 |
| [39] | Wang M, Lloyd G T, 2016. Rates of morphological evolution are heterogeneous in Early Cretaceous birds. Proc R Soc B-Biol Sci, 283: 20160214 |
| [40] | Wang X R, Shen C Z, Liu S Z et al., 2015. New material of Longipteryx (Aves: Enantiornithes) from the Lower Cretaceous Yixian Formation of China with the first recognized avian tooth crenulations. Zootaxa, 3941: 565-578 |
| [41] |
Wilkinson D M, Ruxton G D, 2012. Understanding selection for long necks in different taxa. Biol Rev, 87: 616-630
DOI URL |
| [42] |
Wu Y, 2021. Molecular phyloecology suggests a trophic shift concurrent with the evolution of the first birds. Commun Biol, 4: 547
DOI PMID |
| [43] |
Xu X, Zhou Z H, Wang Y et al., 2020. Study on the Jehol Biota: recent advances and future prospects. Sci China Earth Sci, 63: 757-773
DOI |
| [44] |
Zelenkov N V, 2017. Early Cretaceous Enantiornithine birds (Aves, Ornithothoraces) and establishment of the Ornithuromorpha morphological type. Paleontol J, 51: 628-642
DOI URL |
| [45] |
Zelenkov N V, Averianov A O, 2016. A historical specimen of enantiornithine bird from the Early Cretaceous of Mongolia representing a new taxon with a specialized neck morphology. J Syst Palaeontol, 14: 319-338
DOI URL |
| [46] |
Zhang F C, Zhou Z H, Hou L H et al., 2001. Early diversification of birds: evidence from a new opposite bird. Chinese Sci Bull, 46: 945-949
DOI URL |
| [47] |
Zheng X T, O’Connor J K, Huchzermeyer F et al., 2014. New specimens of Yanornis indicate a piscivorous diet and modern alimentary canal. PLoS One, 9: e95036
DOI URL |
| [48] |
Zhou S, O’Connor J K, Wang M, 2014a. A new species from an ornithuromorph (Aves: Ornithothoraces) dominated locality of the Jehol Biota. Chinese Sci Bull, 59: 5366-5378
DOI URL |
| [49] |
Zhou S, Zhou Z H, O’Connor J K, 2014b. A new piscivorous ornithuromorph from the Jehol Biota. Hist Biol, 26: 608-618
DOI URL |
| [50] |
Zhou Z H, Zhang F C, 2002. A long-tailed, seed-eating bird from the Early Cretaceous of China. Nature, 418: 405-409
DOI URL |
| [51] |
Zhou Z H, Zhang F C, 2005. Discovery of an ornithurine bird and its implication for Early Cretaceous avian radiation. Proc Nat Acad Sci USA, 102: 18998-19002
DOI URL |
| [52] |
Zhou Z H, Zhang F C, 2007. Mesozoic birds of China-a synoptic review. Front Biol China, 2: 1-14
DOI URL |
| [53] |
Zhou Z H, Clarke J, Zhang F C et al., 2004. Gastroliths in Yanornis: an indication of the earliest radical diet-switching and gizzard plasticity in the lineage leading to living birds? Naturwissenschaften, 91: 571-574
DOI URL |
| [54] |
Zhou Z H, Clarke J, Zhang F C, 2008. Insight into diversity, body size and morphological evolution from the largest Early Cretaceous enantiornithine bird. J Anat, 212: 565-577
DOI PMID |
| [55] | Zhou Z H, Zhang F C, Li Z H, 2009. A new basal ornithurine bird (Jianchangornis microdonta gen. et sp. nov.) from the Lower Cretaceous of China. Vert PalAsiat, 47(4): 299-310 |
| [56] |
Zhou Z H, Meng Q, Zhu R et al., 2021. Spatiotemporal evolution of the Jehol Biota: responses to the North China craton destruction in the Early Cretaceous. Proc Nat Acad Sci USA, 118: e2107859118
DOI URL |
| [1] | ZHOU Ya-Chun, Corwin SULLIVAN, ZHANG Fu-Cheng. Negligible effect of tooth reduction on body mass in Mesozoic birds . Vertebrata Palasiatica, 2019, 57(1): 38-50. |
| [2] | LIU Di, ZHOU Zhong-He, ZHANG Yu-Guang. MASS ESTIMATE AND EVOLUTIONARY TREND IN CHINESE MESOZOIC FOSSIL BIRDS . Vertebrata Palasiatica, 2012, 50(1): 39-52. |
| [3] | Benjamin H. PASSEY*, Jussi T. ERONEN, Mikael FORTELIUS , ZHANG Zhaoqun . Paleodiets and paleoenvironments of Late Miocene gazelles from North China: Evidence from stable carbon isotopes . Vertebrata Palasiatica, 2007, 45(2): 118-127. |
| [4] | Diana PUSHKINA. Dynamics of the mammalian fauna in southern siberia during the late palaeolithic . Vertebrata Palasiatica, 2006, 44(03): 262-273. |
| [5] | Hou Lianhai, Zhou Zhonghe, Gu Yucai, Sun Yutie. INTRODUCTION TO MESOZOIC BIRDS FROM LIAONING, CHINA . Vertebrata Palasiatica, 1995, 33(04): 261-271. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||